Each device that is intended to be distributed in the Untied States must have a device listing. Registration and Device Listing go hand in hand. Below is a sample device listing for a particular product type that is offered by the establishment.
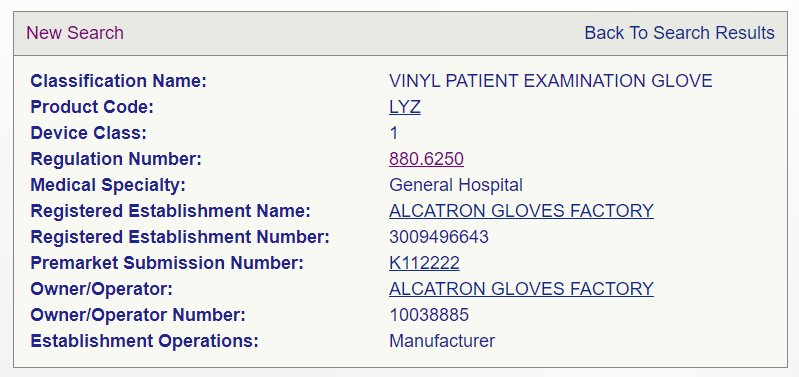
Important Listing Points
- Accurately Classify the Product. It is critically important that the device classification is accurate. The classification drives premarket approval or exemption, import clearance by customs and the labeling requirements for the product.
- Check Similar products. Determine classification based on a similar device. Check the indented use of the device and select the appropriate product code.
- Select the Product Code. The product code brings in the the class and regulation used to qualify the device. If the regulation states the product requires premarket approval, then the 510(k) process must be initiated to obtain FDA clearance for the device before it can be imported.
- Identify labeling issues that need to be fixed. For example there is a big difference between a Level 1 or Level 2 Isolation gown and an Level 3 Isolation Gown or a Surgical Gown. The Level 3 and above or “Surgical” gowns require 510(k) clearance because they are used in more high risk area. The labeling language can trigger that requirement.







